In this section
Gustavo J. Martinez, PhD

Microbiology and Immunology Discipline
Center for Cancer Cell Biology, Immunology, and Infection
Gustavo Martinez earned his MSc degree at the University of Buenos Aires, School of Natural and Exact Sciences in Argentina. He studied the role of costimulatory molecules on T cells in patients with Mycobacterium infections (tuberculosis and leprosy).
In 2006, Gustavo moved to the United States to earn his PhD in the laboratory of Dr. Chen Dong. Gustavo’s research at the time focused on understanding the molecular mechanisms underlying the transcriptional regulation of T helper 17 and regulatory T cells. In particular, he focused on the role of the Treg master transcription factor Foxp3 in Th17 cell differentiation, and the importance of the Smad-dependent branch of TGF-beta signaling in Th17/Treg differentiation. He also collaborated in other projects elucidating the mechanisms driving Th17, regulatory T and follicular helper T cells generation.
In 2011, Dr. Martinez joined the laboratory of Dr. Anjana Rao and Dr. Patrick Hogan. As a Jane Coffin Childs Memorial Fund fellow, Dr. Martinez investigated the role of the Nuclear Factor of Activated T cell (NFAT) family of transcription factors in both CD4 and CD8 T cell responses to viral infection, as well as the role of NFAT in the generation of hyporesponsive states in T cells upon deficiency in binding to its partner AP-1 transcription factor. Dr. Martinez showed that NFAT transcription factors can drive the generation of follicular helper T cells upon acute viral infection, and also demonstrated an important role of NFAT1 in the generation of exhaustion-associated genes.
RESEARCH INTERESTS
The overall goal of the laboratory is to understand the transcriptional and epigenetic changes occurring during the activation and differentiation of T lymphocytes. The following research projects are ongoing in the laboratory:
* Crucial role of NFAT family members in T cell exhaustion. In the face of chronic infections (persistent antigen stimulation), antigen-specific CTLs show a gradual decrease in effector function, a phenomenon that has been termed CD8+ T cell “exhaustion”. CD8+ T cell exhaustion has been observed in patients with HIV, HBV, and HCV infections, as well as in several forms of cancer. While the phenotype of these cells has been described, the molecular mechanisms that lead to their generation remain unclear. I showed that NFAT transcription factors play important roles in CD8+ T cell exhaustion.
I found that NFAT family members are crucial for induction of several exhausted-associated genes, using RNA-seq and ChIP-seq approaches as well as in vivo bacterial infection and tumor models (Martinez GJ et al, 2015). These results highlight the importance of NFAT family members not only in T cell activation but also in the generation of hyporesponsive states, which need to be reverted in chronic infections and in anti-tumor responses. Our studies are now focused at understanding how NFAT transcription factors, by cooperating with other transcriptional regulators, can achieve gene regulation specificity.
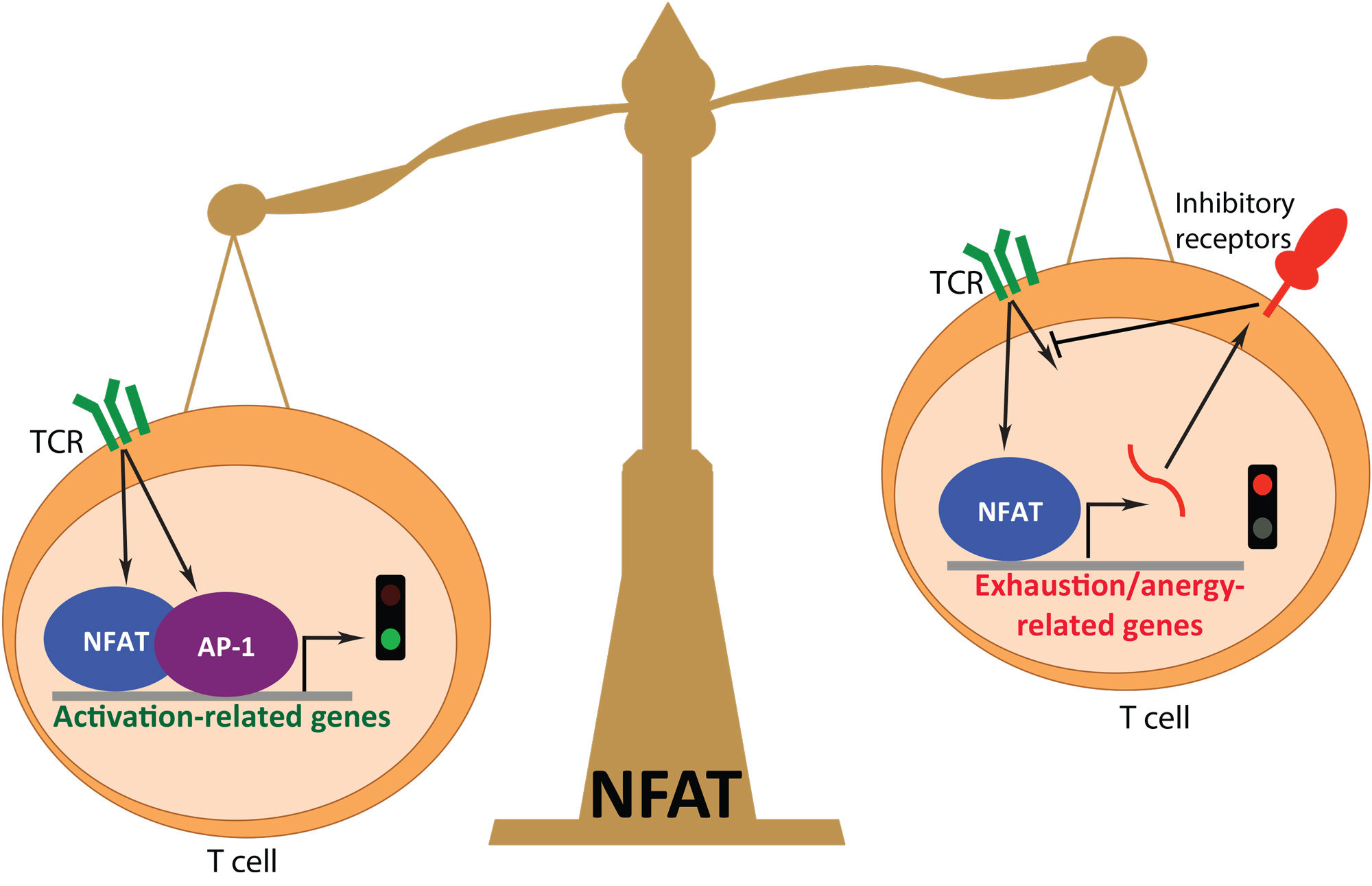
* Role of NFAT transcription factors in CTL differentiation and function. CD8+ T cell differentiation orchestrated by transcription regulators is critical for balancing pathogen eradication and long-term immunity by effector and memory CTLs, respectively. The transcription factor Nuclear Factor of Activated T cells (NFAT) family members are known for their roles in T cell development and activation but still largely undetermined in CD8+ T cell differentiation in vivo. We found that NFAT1 is critical for effector population generation whereas NFAT2 is required for promoting memory CTLs in a cell intrinsic manner (Xu et al. 2019). Importantly, mice lacking both NFAT1 and NFAT2 in T cells displayed an additive effect, were unable to clear an acute viral infection.
NFAT-deficient CTLs showed different degrees of impaired IFN-γ and TNF-α expression with NFAT1 being mainly responsible for IFN-γ production upon ex-vivo stimulation as well as for antigen-specific cytotoxicity. Our results suggest that NFAT1 and NFAT2 have distinct roles in mediating CD8+ T cell differentiation and function. Further research is aimed at further identifying the specific functions of each family member during viral infections.

* Epigenetic regulation of T cells. While there has been much focus on the transcriptional regulation of T cell differentiation, the cell-intrinsic changes at the epigenetic level are less fully understood. Epigenetic regulation includes DNA methylation, long-non-coding RNA interaction, and covalent modifications of histone residues. Histone modifications such as acetylation, methylation, sumoylation, phosphorylation and ubiquitination play important roles in regulating gene expression. While chromatin dynamics of naïve T cell activation are directly associated with transcriptional control of gene expression and T cell lineage commitment, the exact molecular mechanisms governing this process are still elusive.
In particular, the lab is focused on understanding methylation regulation of histone residues and its impact on gene expression, differentiation and function. We recently demonstrated that Lysine Demethylase 6b (Kdm6b), through induction of chromatin accessibility in key effector-associated gene loci, allows for the proper generation and function of effector CD8 T cells in vivo (Xu et al. 2021).

PUBLICATIONS
Complete list of Dr. Martinez' publications
Most relevant publications:
Xu Tianhao, Schutte A, Jimenez L, Gonçalves ANA, Keller A, Pipkin ME, Nakaya HI, Pereira RM, and Martinez GJ. Kdm6b regulates the generation of effector CD8+ T cells by inducing chromatin accessibility in effector-associated genes. J. Immunol. 2021. 2021 May 1;206(9):2170-2183
Xu T, Keller A, and Martinez GJ. NFAT1 and NFAT2 Differentially Regulate CTL Differentiation Upon Acute Viral Infection. Front Immunol. 2019 Feb 15;10:184.
Tanaka K*, Martinez GJ*, Yan X, Long W, Ichiyama K, Chi X, Kim BS, Reynolds JM, Chung Y, Tanaka S, Liao L, Nakanishi Y, Yoshimura A, Zheng P, Wang X, Tian Q, Xu J, O’Malley BW, and Dong C. Regulation of pathogenic T helper 17 cell differentiation by Steroid Receptor Coactivator-3. Cell Reports. 2018. May 22; 23(8):2318-2329.
* co-first authorship.
Pereira RM#, Hogan PG, Rao A, Martinez GJ#. Transcriptional and epigenetic regulation of T cell hyporesponsiveness. J. Leukoc. Biol. 2017; 102(3):601-615.
# corresponding author.
Martinez GJ#, Hu JK, Pereira RM, Crampton JS, Togher S, Bild N, Crotty S, and Rao A. Cutting Edge: NFAT transcription factors promote the generation of follicular helper T cells in response to acute viral infection. J. Immunol. 2016. Mar 1;196(5):2015-9. # corresponding author.
Martinez GJ*, Pereira RM*, Äijö T*, Kim EY, Marangoni F, Pipkin ME, Togher S, Heissmeyer V, Zhang YC, Crotty S, Lamperti ED, Ansel KM, Mempel TR, Lähdesmäki H, Hogan PG, and Rao A. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 2015 Feb 17;42(2):265-78.
Martinez GJ, Zhang Z, Reynolds JM, Tanaka S, Chung Y, Liu T, Robertson E, Lin X, Feng XH and Dong C. Smad2 positively regulates the generation of Th17 cells. J. Biol. Chem. 2010. 285(38):29039-43.
Martinez GJ, Zhang Z, Chung Y, Reynolds JM, Lin X, Jetten A, Feng XH and Dong C. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J. Biol. Chem. 2009. 284(51):35283-6.
Martinez GJ, Nurieva RI, Yang XO and Dong C. Regulation and function of pro-inflammatory TH17 cells. Ann. N. Y. Acad. Sci. 2008. 1143, pp. 188-211.
Yang XO*, Nurieva R*, Martinez GJ*, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008. 29(1):44-56.
